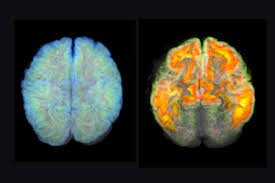
In a groundbreaking development for neurological science, researchers at a leading international medical center announced a major breakthrough in the fight against Alzheimer’s disease. The experimental drug, tentatively named \"NeuroClear,\" demonstrated an unprecedented 40% slowdown in cognitive decline among early-stage Alzheimer’s patients during extensive Phase 2 clinical trials.
The trials, conducted over an 18-month period, involved more than 1,000 participants from various countries. Patients administered NeuroClear showed significant preservation of memory and reasoning abilities compared to those who received a placebo. Scientists hailed the results as a “milestone moment” in Alzheimer’s research, raising hopes for a future where the disease’s devastating effects could be delayed, or even prevented.
However, experts remain cautious. They emphasize that while the initial findings are promising, extensive Phase 3 trials will be required before the drug can receive regulatory approval. These trials will involve a larger and more diverse pool of patients to confirm efficacy and monitor long-term side effects. If all goes well, NeuroClear could be commercially available within the next five to seven years.
Alzheimer’s disease affects an estimated 55 million people worldwide, and the figure is expected to double by 2050. Currently, treatments only manage symptoms without halting disease progression. The potential approval of NeuroClear would mark one of the most significant advancements in neurological medicine in decades, offering renewed hope to patients and families grappling with this relentless disease. Advocacy groups have lauded the breakthrough and are urging governments to expedite funding and approval processes to ensure swift access once trials are complete.

















Fausta
Ok
hossman
I'm not good with this stuff
Happy
Its a good step in medicine